- Bayi
- Alat Permainan & Lain Lain
- Pakaian Kanak-Kanak Lelaki
- Kasut Kanak-Kanak Lelaki
- Beg Kanak-Kanak Lelaki
- Aksesori Kanak-Kanak Lelaki
- Pakaian Kanak-Kanak Perempuan
- Kasut Kanak-Kanak Perempuan
- Beg Kanak-Kanak Perempuan
- Aksesori Kanak-Kanak Perempuan
- Penjagaan Bersalin
- Produk Asuhan Anak-Anak
- Pakaian Bayi
- Pemakanan Bayi
- Mandian & Perapian
- Diapering & Potty
- Gear Bayi
- Keselamatan & Kesihatan
- Pakaian Unisex
- Penjagaan Peribadi Bayi
- Beg Unisex
- Keperluan Sekolah
- Pakaian Luaran
- Pakaian
- Skirt
- Seluar & Legging
- Jumpsuits & Playsuits
- Pakaian luar & Kardigan
- Pakaian Tradisional Wanita
- Pakaian Hamil
- Saiz besar
- Bikini Wanita
- Pakaian Sukan Wanita
- Pakaian Dalam, Tidur & Bersantai
- Beg & Dompet Wanita
- Kasut wanita
- Jam Tangan Wanita
- Cermin Mata Wanita
- Perhiasan Fesyen Wanita
- Pakaian muslimah
- Aksesori wanita
- Baju Formal Wanita
- Set Pakaian Kasual (Baju & Seluar)
- Uniform
- Fashion Gift
- Pencuci Barang Kemas
- Handsocks & Socks
- Aksesori Telefon Bimbit
- Aksesori Tablet
- Aksesori Komputer / Komputer riba
- Komponen Komputer
- Aksesori Kamera
- Aksesori Jam Tangan Pintar
- Aksesori Permainan
- Peranti Pintar
- Fon kepala & alat dengar
- Alat dengar tanpa wayar
- Earbud Tanpa Wayar
- Pembesar suara tanpa wayar
- Peti TV Android
- Mikrofon tanpa wayar
- Earbuds
- Network Components
- Alat Bakeware & Baking
- Kopi & Teh
- Peralatan memasak
- Peralatan makan
- Gelas
- Linen Dapur
- Alat Dapur
- Penyimpanan Makanan
- Sink Organisasi
- Serveware
- Yang lain
- Penyimpanan & Aksesori Dapur
- Peralatan dapur
- Lekapan Dapur
- Penyusun Dapur
- Rak dapur
- Penyimpanan & Aksesori Dapur
- Tisu
- Tisu tandas
- Pencuci pinggan
- Cecair Cucian buah-buahan & sayur-sayuran
- Perabot bilik tidur
- Perabot Ruang Tamu
- Perabot Kanak-kanak & Bayi
- Hallways & Entry Furniture
- Perabot Permainan
- Perabot pejabat
- Taman
- Aksesori Bilik Mandi
- Aksesori Tempat Tidur
- Hiasan rumah
- Lampu
- Alatan tangan
- Power Tools
- Keselamatan
- Hardwares & Components
- Plumbing & Flooring
- Shelving & Garage Storage
- Stationery
- Seni dan kraf
- Gift & Wrapping
- Produk Kertas
- Kraftangan Malaysia
- Muzik & Instrumen
- Perabot Makan
- Perabot Dapur
- Elektrik
- Peralatan Pembersihan & Pembersihan
- Semburan
- Tisu muka
- Kalendar
- Buku Inggeris
- Chinese Books
- Buku Bahasa Melayu
- Bahan Bacaan Lain
- Buku teks
- Local Book
- Educational Book
- Religious Book
- Psychology & Relationships
- Action, Crime & Thrillers
- Comics & Manga
- History & Cultures
- Children's Books
- Recipes & Cooking
- Business & Investment
- Careers, Self Help & Personal Development
- Travel & Tourism
- Politics, Law & Social Sciences
- Health, Fitness & Dieting
- Fiction book
- Music Books
- Collectibles & Memorabilia
- E-Buku
- Berus & Set Makeup
- Celak
- Bulu Mata
- Gincu
- Pengilat bibir
- Pensel bibir
- Maskara
- Pemerah pipi
- Concealer
- Bedak asas
- Primer, Balm & Perapi
- Pembersih solekan
- Bedak kompak
- Pengilat pipi
- Kening
- Celak mata
- Palet & Set
- Pewarna kuku
- Kit penjagaan kuku
- Tempat penyimpan alat solek
- Aksesori solek
- Pengilat kuku
- Lip Tint
- Seting & Finishing Spray
- Multivitamin
- Khasiat Kulit
- Penyekat & Pembakar Lemak
- Imunisasi
- Minda & Ingatan
- Suplemen Sukan
- Detoksifikasi
- Tekanan Jantung & Darah
- Penggantian Makanan
- Penambah Berat
- Produk Pemutih
- Makanan Kecantikan
- Makanan & Minuman Berkhasiat
- Minyak
- Kesihatan
- Kesihatan Lelaki
- Penjagaan Penglihatan
- Kesihatan wanita
- Herba & Perubatan Tradisional
- Bahan Koleksi & Tokoh Aksi
- Mainan & Bangunan Mainan
- Mainan Muzik
- Mainan Pendidikan
- Mainan Kenderaan & Alat Kawalan Jauh
- Mainan Imaginasi
- Mainan Luaran
- Anak Patung
- Mainan Bayi & Kanak-Kanak
- Mainan Perkembangan Awal
- Pembacaan & Penulisan
- Seni & Kraf
- Anak Patung & Rumah Anak Patung
- Teka-teki & Permainan Papan
- Lain-Lain
- Battling Tops
- Mainan Hobi
- Periuk nasi
- Pengisar
- Pengadun & Aksesori
- Periuk Elektrik Bertekanan Tinggi
- Periuk Perlahan
- Periuk pelbagai fungsi
- Cooktops & Ranges
- Electric Kettle & Thermo Pot
- Pembuat Roti, Pembakar roti, Wafel & Sandwic
- Pembuat Ais Krim
- Pembuat jus & buah
- Air Fryer & Deep Fryer
- Mesin kopi
- Pengukus Makanan Elektrik
- Pemanas Ketuhar & Oven
- Alat Panggang Elektrik
- Perkakas Masakan Khas
- Deco lampu
- Lampu Siling
- Lampu latar
- Lampu Candelier
- Lampu loket
- Lampu Spot
- Lampu Meja
- Lampu Dinding
- Mentol Lampu LED
- Mentol Pendarfluor
- Lampu Trek
- Lampu Pintu Luar
- Lampu loket luar
- Lampu Dinding Luar
- Lampu Langkah
- Lampu Lonjakan
- Lampu Banjir
- Yang lain
- LED Tube
- Floor Lamps
- Specialty Lighting
- Outdoor Lighting
- Desk Organisers
- Pencil Cases & Boxes
- School Sets
- Filing & Document Presentation
- Boards & Flipcharts
- Calculators
- Pita & Dispenser
- Staplers & Punches
- Gunting & Pemotong
- Pen
- Highlighters & Markers
- Pensel
- Dakwat & Isi Ulang
- Yang lain
- Penjimatan Wang
- Pita Pembetulan & Kertas Cecair
- Gam
- Colour Pencil
- Crayon
- Colour Pen
- Pensil mekanikal
- Jual Dengan Kami
-
Imbas Kod QR untuk memuat turun aplikasi PGMall ke Telefon anda.
-
-
Notifikasi
Notifikasi BaharuLog masuk untuk melihat NotifikasiAnda tidak notifikasi baruDaftar atau Log Masuk
- Help
- Daftar
- Log Masuk
- Home
- Health & Beauty
- Medical Supplies
- Health Monitors and Tests
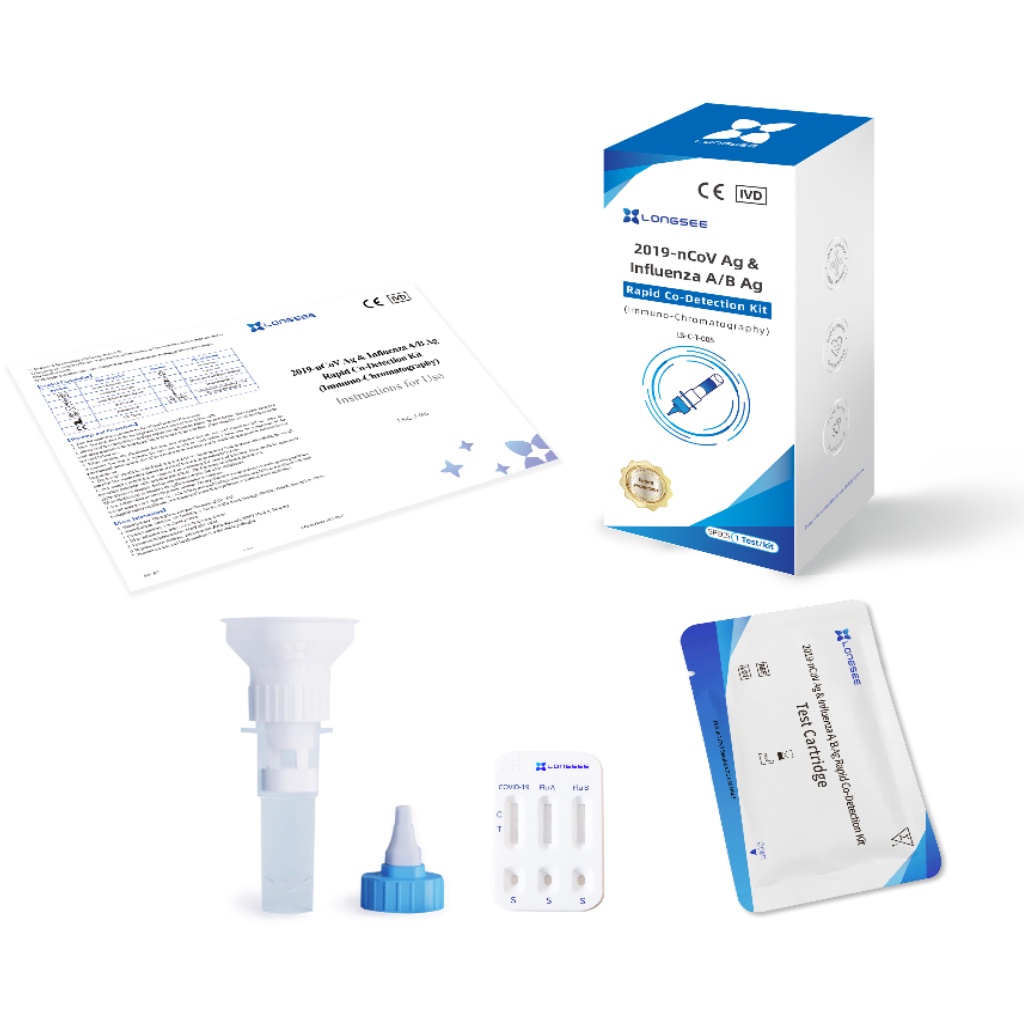
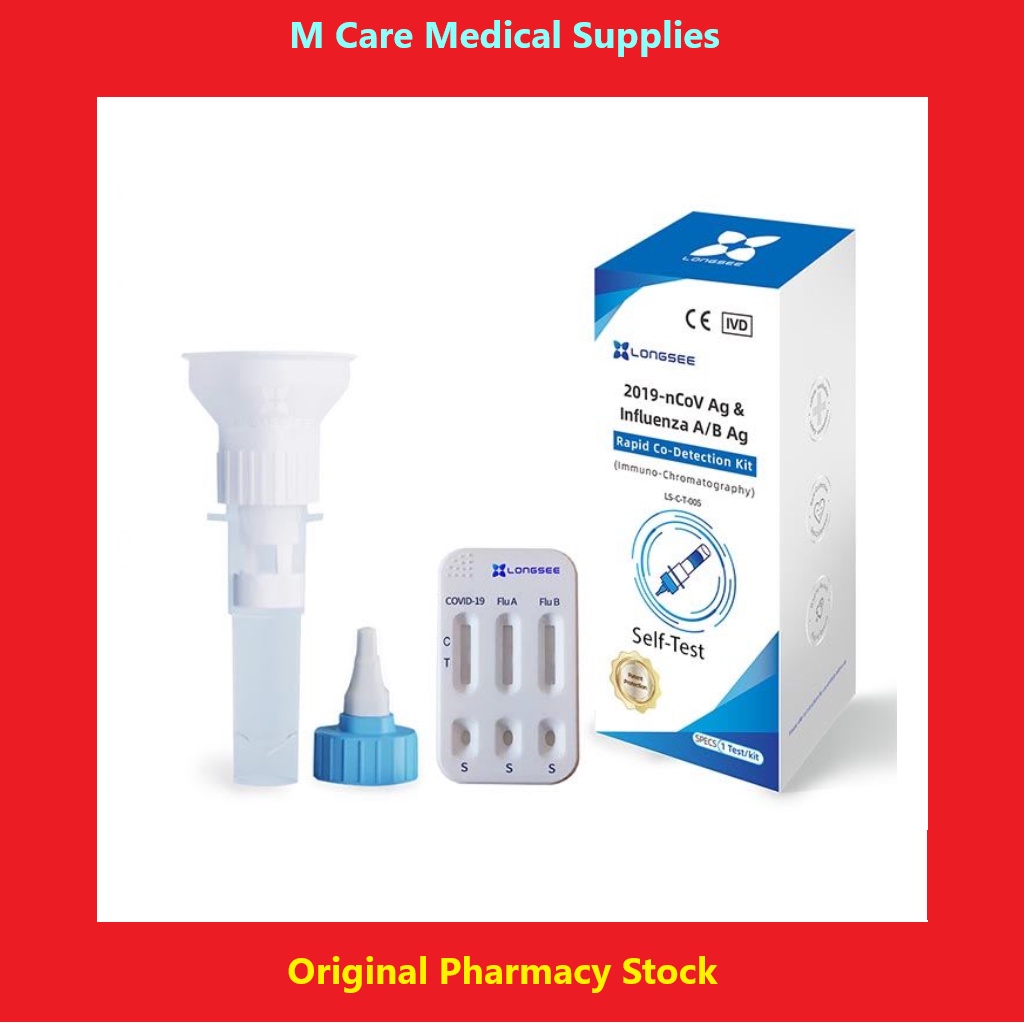
- Longsee 2019-nCoV Ag & Influenza A/B Ag Rapid Co-Detection Kit


Penghantaran
Warna
Kuantiti
RM 40.27
Model :LS-C-T-005
Intended Use :2019-nCoV Ag & Influenza A/B Ag Rapid Co-Detection Kit(Immuno-Chromatography)is an immunochro-matographic assay for rapid, qualitative detection of influenza A/B antigen and novel coronaviruses (2019-nCoV) antigen extracted from the saliva specimen. The test is to be used as an aid in the diagnosis of influenza A/B and coronavirus infection disease (COVID-19). The test provides preliminary test results. Negative results cannot exclude influenza A/B and 2019-nCoV infection and they cannot be used as the sole basis for treatment or other management decision. For in vitro diagnostic use only. For professional use only.
Storage Condition & Expiration Date
1) 12 months at 4-35 °C.
2) Do not freeze. The test card should be used as soon as possible within 30 minutes after the aluminium foil bag is opened.
Test Procedure
1. Allow all kit components to equilibrate to room temperature (15-30°C) prior to testing for 30 minutes.
2. Do not place anything in the mouth including food,drink,gum, tobacco,water and mouthwash products for at least 30 minutes prior to collection of oral fluid specimen.
Specimen Collection & Extraction
1. Spit the expectorated saliva into the collection funnel gently as much as possible close to 1mL.
2. Unscrew the collection funnel gently to allow the saliva flow into the collection tube completely.
3. Screw the nozzle onto the collection tube.
4. Turn upside down the collection tube slightly for 5 seconds, so that the diluent can mix with the saliva sample evenly.
5. Leave the collection tube stand at room temperature for 5 minutes.
6. Remove the nozzle cap.
Reaction with Test Cartridge
1. Remove a test cartridge from the sealed pouch by tearing at the notch and place it on a level surface.Drip 3~4 drops (about 100 μL) of extracted specimens vertically into the specimen well (S) on the test cartridge by squeezing the collection tube. The formation of air bubbles in the specimen well (S) must be avoided.Do not handle or move the test cartridge until the test is complete and ready for reading.
2. Start timer. Read result within 15 ~20 minutes of adding the sample. The test result is invalid after 20 minutes.
Interpretation of the Result
Invalid: When there is no red line in the quality control area (C), the test is invalid. It is recommended to retest with a new test card, paying particular attention to whether the sample volume is sufficient.
Positive: A red line appears in the detection area (T) and the quality control area (C) of the lane COVID-19,Flu A and Flu B respectively.
Negative: A red line only appears in the quality control area (C).

Hair Dryer Brush, Hot Air Brush One Step Hair Dryer and Styler & Volumizer 3 IN 1 Negative Ions Dryer Brush with Straigh
RM 111.54
RM 55.77

🌿 ESSENTIAL | Magnesium Oil 100ml x 1
RM 111.80
RM 55.90

(Towel Rack) Rak Hanger Holder Hanging Towel Bar Layer Bathroom Toilet Kitchen Wall Mounted Gantung Tuala Tandas 浴室架 毛巾架
RM 90.74 - RM 120.90
RM 45.37 - RM 60.45

KT WARE Juice Tower Bekas Air Menara Balang Air Tabung Air Jug air Beer Tower.
RM 168.74 - RM 246.74
RM 84.37 - RM 123.37

Kuali Honeycomb Wok Stainless Steel 304 Nonstick Frypot Cooking Frying Pan Kuali Wok Cookware Masak
RM 150.80
RM 75.40 - RM 150.80

Cosway Powermax Dish Drops bathroom cleaner sets
-
RM 78.00